About the client
Our client operates manufacturing and research facilities across India, the US, and Brazil, with a strong presence in regulated markets like the US and Europe, as well as key regions in Latin America and South Africa.
Known for their comprehensive healthcare solutions, they offer a wide range of products from pharmaceuticals to animal healthcare. With a legacy dating back to the 1950s, they have grown significantly, focusing on innovation and addressing unmet healthcare needs globally.
Challenges
▪ The chemical / Impurities / Reference standard items were difficult to differentiate.
▪ Duplicate identification of spare items.
Business Need
Support in Designing, sustaining & improving the systems for master data management.
To ensure timely review, validation, and updating of the master data.
To ensure continuous knowledge transfer and training to all Plant users.
Project Scope
With our Master Data Management tool, Prosol:
1. Validating and Maintaining the description as per Naming convention
2. Correcting Material Type, Material Group as per type of material
3. Checking CIN data is available for all requests.
4. Checking correct UOM as per ordering and issuing of material.
5. Perform a 100 % duplicate check to find out and eliminate the addition of multiple records for existing items in daily creation.
6. Review and Update the UNSPSC in system
7. Execute the description changes requested by the Plant
8. Change the correct UOM as requested by the GDSO
9. Blocking the material as requested by GDSO
10. Support and rectification of ICS audit points for the plant user
11. Doing Amendments and Creating material codes upon telephonic request
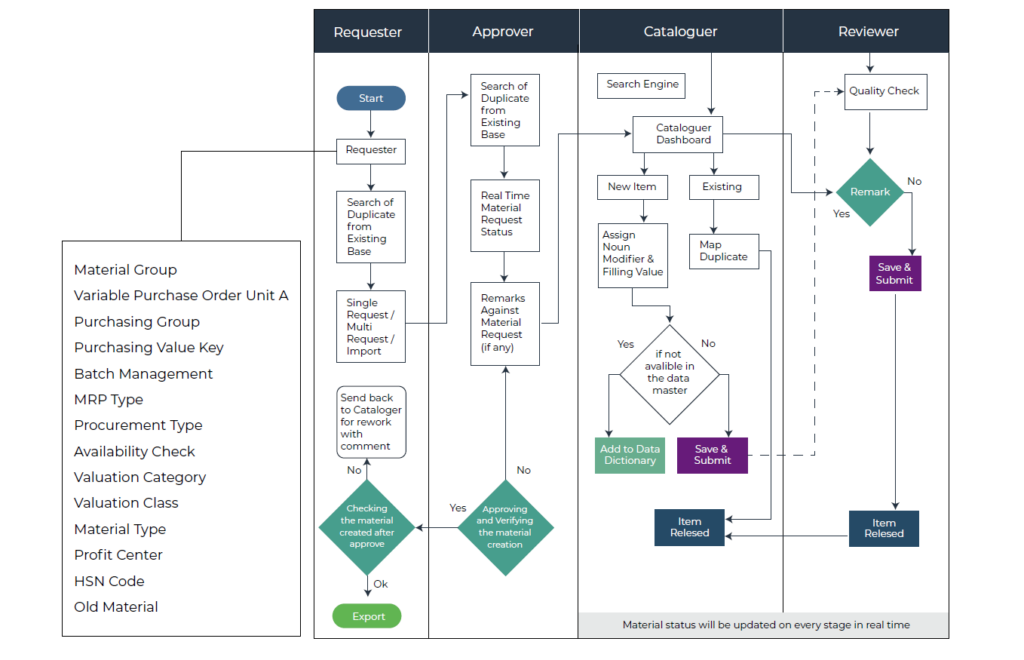
Work Strategy
Material Master Workflow – Prosol